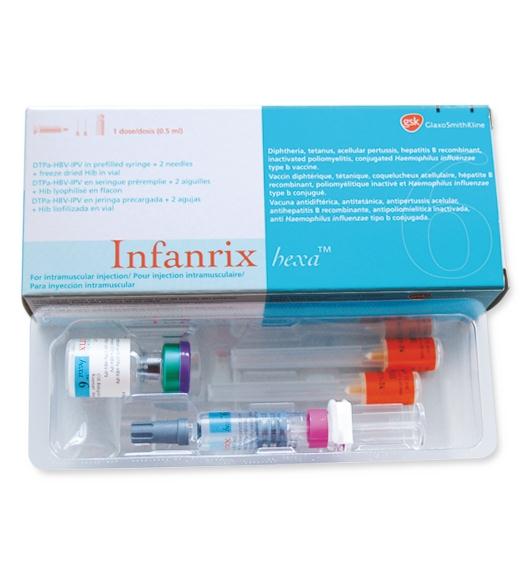
GlaxoSmithKline (GSK) is recalling Batch A21CD706B of Infanrix Hexa injectable vaccine.
Infanrix Hexa is a vaccine given to infants to provide protection against Diphtheria, Tetanus, Pertussis, Hepatitis B, Poliomyelitis and diseases caused by Haemophilus influenzae Type B.
The recalled vaccines were stored under inappropriate storage (temperature) conditions during importation, which can affect the stability of the vaccine components, thus reducing the effectiveness of the vaccine, a release from the Ministry of Health Stated on Saturday.
It stated, “Out of an abundance of caution, the Ministry of Health is therefore informing the public of this voluntary recall.”
It added, “It is further noted that this vaccine is not used in the public health sector and hence the recall is confined to private medical facilities and at private doctors’ offices.”

The Ministry of Health advised that parents who believe their children may have received the Infanrix Hexa Injectable (Batch A21CD706B) vaccine should immediately contact their doctor for advice and follow up patient care, as required.
The release staed, “Also, doctors should contact the parents of their patients who were administered with the affected batches and provide the necessary care.”

Additional information can be obtained by contacting the Office of the Director of Chemistry, Food and Drugs Division at 868- 623-5242 /800-CFDD.
![]()