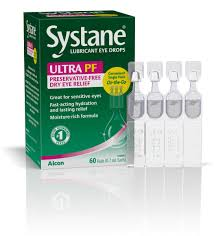
THE Ministry of Health has announced a voluntary recall of a specific batch of Systane Lubricant Eye Drops Ultra PF, Single Vials On-the-Go, following a contamination report.
The recall, issued by Alcon Laboratories, affects lot 10101 of the product, which is used to relieve dry eye symptoms such as burning and irritation.
The recall was initiated after foreign material, identified as fungal, was discovered in a sealed single-use vial.
Alcon Laboratories warns that fungal contamination in eye products can potentially cause eye infections, posing a risk of vision-threatening conditions and, in very rare cases, could be life-threatening for immunocompromised individuals.
However, the company has not received any reports of adverse events linked to this recall.
The local distributor has assured that no products affected by this batch were imported into Trinidad and Tobago.
Despite this, the Ministry advises anyone in possession of the affected batch to stop using the product immediately and return it to the place of purchase if possible.
For further information, individuals can contact the Office of the Director of the Chemistry, Food and Drugs Division via email at cfdd@health.gov.tt or by phone at 217-4664 ext. 13101.
![]()