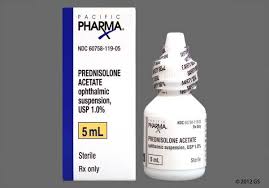
The Ministry of Health (MoH), through its Chemistry, Food and Drugs Division, has issued a public advisory regarding a voluntary recall notice for Prednisolone Acetate Ophthalmic Suspension USP 1% w/v 5ml Eye Drops.
The recall, initiated by the manufacturer, Juvencia Lifesciences Private Limited, comes in light of potential bacterial contamination concerns.
In a release on Wednesday, the ministry stated that Prednisolone Eye Drops are commonly prescribed for the treatment of non-infectious eye allergies and inflammation. However, the Ministry urges caution as the affected batches, totaling 8,000 packs, bear the Batch Number N23B27.
In light of the recall, individuals in possession of the recalled product are strongly advised to cease its usage immediately. Furthermore, the Ministry recommends returning the product to the point of purchase or the location where it was received, whenever feasible.
For further inquiries or assistance regarding the recall, individuals can reach out to the Office of the Director of the Chemistry, Food and Drugs Division. They can be contacted via email at cfdd@health.gov.tt or by phone at 217-4664 ext. 13101.

Support independent journalism. AZP News is an independent news organisation that is not affiliated with any big business and depends solely on advertising to pay our bills. Therefore, we are asking for the generosity of our readers to help us with small donations of any amount, but we will be happy with $20, $50 or $100. Click Here to Donate
![]()